Welcome To ChemAnalyst
This blog delves into the industrial production of Sorbitol, focusing on the catalytic hydrogenation of glucose derived from starch sources like corn and cassava. It outlines various process technologies, key catalysts used, and downstream applications. Additionally, it discusses the importance of raw material sourcing, quality standards, and emerging trends like green chemistry and enzymatic production. The article serves as a valuable resource for understanding the industrial economics and sustainability challenges in Sorbitol manufacturing.
Introduction
Sorbitol is a sugar alcohol derived from glucose and is a used as an ingredient in the pharmaceutical, food & beverage, personal care and nutraceutical industries. It acts as a low-calorie sweetener, texturizer, humectant and excipient in numerous formulations. It is essential to understand production processes of Sorbitol for manufacturers and stakeholders as it directly influences production costs, emission profiles, scalability and product purity. Evaluating production technologies can identify opportunities for emission reduction, process optimization and eco-friendly manufacturing with increasing sustainability mandates and energy cost concerns globally. This blog provides a comprehensive breakdown of industrial sorbitol production including traditional and emerging techniques, equipment, environmental considerations and sector-specific applications.
Overview of the Production Process
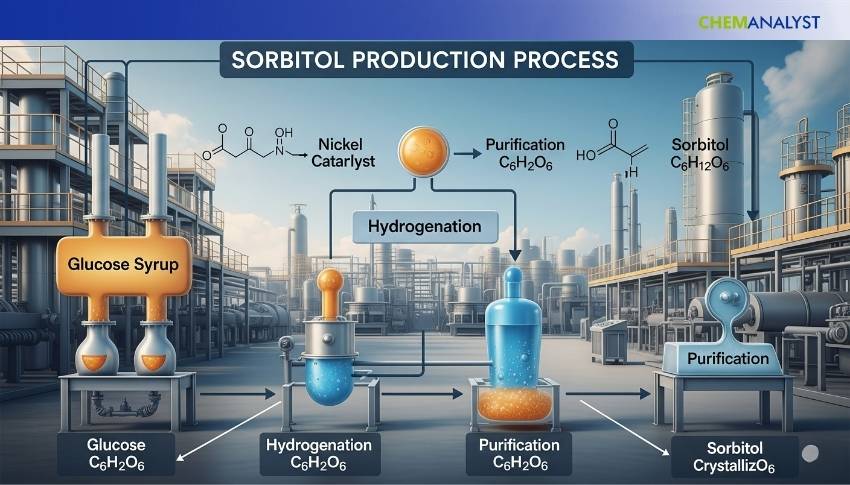
The production of industrial Sorbitol predominantly relies on the catalytic hydrogenation of glucose which is obtained through the hydrolysis of starch-rich crops such as corn, wheat, cassava or potato. The production process can be carried out in either batch or continuous operational setups. The continuous systems have emerged as the preferred option for modern, large-scale and energy-efficient plants. Continuous operations offer consistent product quality, higher throughput, better process control and lower operational costs which makes it highly suitable for industries producing food-grade and pharmaceutical-grade sorbitol.
The production process involves several essential transformation stages. It begins with starch hydrolysis where enzymatic or acid hydrolysis breaks down starch into a glucose-rich syrup. This glucose solution then undergoes a thorough purification process involving filtration, deionization and carbon treatment to eliminate colour impurities, inorganic salts and insoluble residues. The purified glucose syrup is subsequently subjected to catalytic hydrogenation under high-pressure hydrogen gas in the presence of a nickel-based catalyst. This crucial step converts glucose into sorbitol with yields typically exceeding 90% and is accompanied by minor quantities of by-products like mannitol and other sugar alcohols.
The reaction mixture is cooled following hydrogenation and the catalyst is separated often through filtration systems. The resulting crude sorbitol solution is then decolorized using activated carbon and passed through ion-exchange columns to remove residual ionic impurities. The sorbitol solution undergoes vacuum evaporation to concentrate it to a 70% w/w liquid product or is further evaporated, crystallized and dried to produce solid sorbitol in flake, powder or granular forms depending on the desired product form. This multi-stage, energy-optimized and technically refined process ensures high-purity and commercially valuable sorbitol for various end-use sectors.
Major Production Routes
The global production of Sorbitol majorly relies on the well-established catalytic hydrogenation of glucose — which is a mature, efficient and highly scalable process. It is extensively utilized across Asia, Europe and North America. D-glucose is obtained in this method from hydrolysed starch syrups which is subjected to high-pressure hydrogenation in the presence of finely divided nickel-based catalysts, typically supported on silica or alumina. This route is favoured for its exceptional yield efficiency (exceeding 90%), operational reliability and ability to produce both liquid and solid sorbitol grades suitable for a wide range of applications including food, pharmaceuticals, cosmetics and personal care.
The alternate production methods are emerging as niche or sustainable alternatives that are driven by increasing environmental regulations, demand for specialty grades and decentralized production models. The electrochemical reduction of glucose is one such method which involves the use of an electrolytic cell where glucose is reduced at the cathode under mild temperature and ambient pressure conditions. This process eliminates the need for high-pressure hydrogen and metal catalysts and makes it attractive for small-scale or green chemistry setups. However, limitations such as lower selectivity, high energy consumption and limited scalability currently confine its use to academic and pilot-scale research.
The enzymatic bioconversion of glucose is another promising pathway which employs glucose reductase enzymes to catalyse the reduction of glucose to sorbitol under mild aqueous conditions. This biocatalytic process is notable for its high specificity, environmentally friendly profile and potential integration with renewable biomass feedstocks. Though currently constrained by enzyme costs, slow reaction rates and scale limitations, this route is actively pursued for pharmaceutical-grade, high-purity sorbitol and applications where product purity and green credentials are paramount.
Regionally, the catalytic hydrogenation remains the industrial standard worldwide, while electrochemical and enzymatic processes are increasingly explored in Europe, Japan and biotechnology-driven markets for specialty and high-value formulations.
Production Process Details
Catalytic Hydrogenation
Raw Materials and Inputs:
The primary feedstock is D-glucose syrup which is typically obtained from the enzymatic or acid hydrolysis of corn starch, wheat starch, potato starch or cassava starch. This glucose solution must have a high purity (≥98%) to avoid catalyst poisoning. The key catalyst used is finely divided nickel which is supported on inert carriers like silica or alumina. High-purity hydrogen gas is essential for the reduction reaction.
Process & Procedure:
Starch Hydrolysis: Starch is converted into glucose syrup using amylase enzymes or dilute acids under controlled temperature and pH.
Purification: The glucose solution is filtered, deionized and carbon-treated to remove impurities and decolorize the syrup.
Hydrogenation:
• Glucose syrup is introduced into a high-pressure hydrogenation reactor (fixed-bed or stirred tank type).
• Hydrogen gas is supplied at 30–80 bar pressure and 120–160°C.
• In the presence of the nickel catalyst, glucose is hydrogenated to sorbitol.
Post-Reaction Purification: Catalyst is filtered out and the sorbitol solution is decolorized with activated carbon.
Concentration: The purified solution is vacuum evaporated to 70% sorbitol solution or further crystallized and dried for solid grades.
Equipment Used:
• Starch hydrolysis reactors
• Deionizers and carbon columns
• High-pressure hydrogenation reactors
• Catalyst separators and filtration systems
• Ion-exchange units
• Vacuum evaporators and crystallizers
Environmental & Safety Considerations:
• High-pressure hydrogen handling poses explosion risks and requires specialized safety protocols.
• Nickel catalyst disposal must comply with hazardous waste regulations.
• Effluents containing organics and catalyst fines require treatment via biological oxidation and filtration.
• Compliant with EPA guidelines and EU Emission Trading System (EU-ETS) for greenhouse gases.
Electrochemical Reduction of Glucose
Raw Materials and Inputs:
Aqueous glucose solutions (10–20% w/v) serve as the substrate. Electrolytes such as Na2SO4 or H2SO4 are added to enhance conductivity. No hydrogen gas or metal catalysts are needed.
Process & Procedure:
Preparation: Glucose solution is mixed with a suitable electrolyte.
Electrochemical Reaction:
• The mixture is passed into an electrolytic cell with a cathode (lead, platinum, or carbon) and an anode (graphite or platinum).
• Direct current (10–100 mA/cm²) is applied at 20–60°C.
• Glucose is reduced to sorbitol at the cathode while water oxidizes at the anode.
Product Recovery: The resulting sorbitol solution is separated, electrolytes are neutralized or recycled, and by-products like gluconic acid are removed.
Concentration: Vacuum evaporation adjusts sorbitol concentration to commercial levels.
Equipment Used:
• Electrolytic cells (batch or continuous)
• Inert electrodes (platinum, graphite, or nickel)
• DC power supply units with voltage and current control
• Filtration and neutralization systems
Environmental & Safety Considerations:
• High electricity consumption elevates operational carbon footprint.
• Electrode fouling and side reactions (glucose oxidation) generate waste.
• Effluents contain electrolytes requiring neutralization.
• Lower pressure operation reduces explosion risk.
• Preferred for small-scale, specialty or renewable energy-powered operations.
Enzymatic Bioconversion of Glucose
Raw Materials and Inputs:
High-purity glucose solution (≥98%) is the main substrate. The reaction is catalysed by glucose reductase (aldose reductase) enzymes with NADH or NADPH as essential cofactors that are typically regenerated via coupled systems like formate dehydrogenase.
Process & Procedure:
Preparation: Glucose solution is prepared at 15–25% w/v concentration.
Bioconversion Reaction:
• The mixture is introduced into a temperature-controlled bioreactor.
• Glucose reductase and cofactor (or whole-cell catalysts) are added.
• Reaction occurs at 25–40°C and pH 6.0–7.5.
• Cofactor regeneration systems are employed to maintain NAD(P)H levels.
Product Recovery: After several hours, the reaction mixture is filtered to remove enzymes or cells.
Purification & Concentration: The sorbitol-rich solution is decolorized, deionized, and concentrated.
Equipment Used:
• Stirred-tank or membrane bioreactors
• Cofactor regeneration units (formate dehydrogenase systems)
• Temperature and pH control systems
• Activated carbon columns, ion-exchange columns
• Vacuum evaporators for final concentration
Environmental & Safety Considerations:
• Low emissions and mild operating conditions ensure superior safety.
• Biodegradable by-products and enzyme deactivation products require minimal specialized treatment.
• Costly cofactor management systems impact operational expenses.
• Process suits high-purity, pharmaceutical and specialty product markets.
• Limited by enzyme stability, cost and slow reaction rates.
• Excellent fit for green chemistry initiatives and circular bioeconomy models.
Summary Table
Which Process is Used Where?
Catalytic hydrogenation continues to dominate sorbitol production globally with Asia (notably China and India), North America and Europe being major hubs due to superior throughput capacity of the process, consistent product quality and highly optimized infrastructure. This method reliably delivers yields of 90–98% which makes it ideal for producing both food-grade and pharmaceutical-grade sorbitol on a mass scale. Its compatibility with existing high-pressure chemical plants and hydrogenation facilities ensures seamless integration into established manufacturing ecosystems for the food, confectionery, personal care and pharmaceutical industries.
The electrochemical reduction is conceptually attractive for decentralized production models and green electricity integration but still remains confined to academic research, pilot-scale installations and specialty chemical sectors. Its current limitations in conversion efficiency, electrode fouling and operational costs prevent widespread industrial adoption. However, it holds potential for small-batch production of sorbitol derivatives or specialty formulations where access to hydrogen or catalytic systems is restricted in remote pharmaceutical R&D settings or micro-factories aligned with renewable grids.
Enzymatic bioconversion is slower and operationally costlier but excels in markets where product purity, traceability and sustainability metrics are paramount. This includes pharmaceutical-grade sorbitol for intravenous solutions, nutraceuticals and premium personal care formulations. Its mild reaction conditions and highly specific biocatalysts minimize by-product formation and energy consumption which makes it highly suitable for regions prioritizing green bioprocessing such as Western Europe, Japan and select biotech industries in North America. Although commercial-scale enzymatic plants are rare today but the rising demand for eco-certified and bio-based excipients is accelerating its industrial interest.
Conclusion and Future Innovations
Catalytic hydrogenation still remains the industry cornerstone for sorbitol manufacturing not because of environmental pressures, regulatory reforms and growing demand for sustainable excipients but also due to its proven scalability, reliability and adaptability within existing industrial frameworks. However, the technologies are steadily advancing toward greener alternatives. Innovations in enzyme engineering, immobilized biocatalyst systems and efficient cofactor recycling are progressively overcoming the economic and operational limitations of enzymatic bioconversion which are positioning them as an increasingly viable option for high-value, pharmaceutical and specialty applications.
The advancements in electrode materials, electrochemical cell design and integration with renewable electricity infrastructure are revitalizing interest in electrochemical reduction methods. Emerging research into solid-state electrolysers and decentralized modular production units positions electrochemical routes as future-ready solutions for small-scale and on-demand sorbitol production in isolated industrial or pharmaceutical environments.
The shift toward green hydrogen sourcing and nickel catalyst optimization is improving the environmental profile of this dominant process within catalytic hydrogenation itself. The incorporation of hybrid systems that combines catalytic and enzymatic or electrochemical steps for tailored product specifications is also under active investigation.
Sorbitol manufacturers will inevitably need to diversify their production technologies as global sustainability mandates tighten and consumer industries demand cleaner, traceable, and eco-friendly ingredients. This multi-route approach not only ensures operational flexibility and market adaptability but also positions producers to meet both economic goals and stringent environmental compliance standards in the evolving chemical manufacturing landscape.
We use cookies to deliver the best possible experience on our website. To learn more, visit our Privacy Policy. By continuing to use this site or by closing this box, you consent to our use of cookies. More info.
